| Original Article, Biomed Biopharm Res., 2022; 19(2):299-313 doi: 10.19277/bbr.19.2.295; PDF version here [+] ; Portuguese html version [PT] |
Pilot study of unconventional food plant (UFP): adherence to nasturtium (Tropaeolum majus L.) in the diet and monitoring of biometric and clinical indicators
Sérgio Faloni de Andrade1, Maria da Graça Lopes Serrador1, Alda Pereira da Silva1,3,4, Rejane Giacomelli Tavares1,2, Luis Monteiro Rodrigues1, Maria do Céu Costa1,5
1Universidade Lusófona - CBIOS - Research Center for Biosciences and Health Technologies, Av. Campo Grande, 376, 1749-024, Lisboa, Portugal; 2Universidade Federal de Pelotas- PPGNA- Programa de Pós-Graduação em Nutrição e Alimentos, Rua Gomes Carneiro, 01, 96010-610, Pelotas, RS, Brazil; 3Institute for Preventive Medicine and Public Health, Lisbon School of Medicine, University of Lisbon, Portugal; 4Clinic of General and Family Medicine, Ecogenetics and Human Health Unity, Institute for Environmental Health, ISAMB, Portugal; 5NICiTeS, Polytechnic Institute of Lusophony, ERISA-Escola Superior de Saúde Ribeiro Sanches, Lisboa, Portugal
* corresponding author:
Abstract
The flowers of Tropaeolum majus, popularly known as "capuchinha," garden nasturtium, nasturtium, Indian cress, or monks cress, are currently used worldwide as a food supplement and in plant-based diets, being added to smoothies, soups, mayonnaise and salads by consumers in search of sources of substances considered beneficial to human health. Most studies have shown nutritional qualities and beneficial effects of the plant, especially its flowers and respective aqueous extracts in vitro and in animals. Thus, this pilot study was designed to evaluate the acceptance and possible benefits of daily consumption of 20 g T. majus flowers per day for 30 days in healthy participants. Before and after ingestion, body composition, heart rate, blood pressure, and hematological and biochemical parameters were analysed. Results have shown good acceptance and safe use of T. majus flowers in a balanced and varied diet. However, it is important to highlight that this is the first exploratory study regarding these issues in healthy humans, and therefore, despite the widespread consumption described, additional studies are needed to deepen the results in biometric and clinical indicators in a larger number of volunteers.
Keywords: Tropaeolum majus, unconventional food plant (UFP), nasturtium, edible flowers
Received: 13/11/2022; Accepted: 09/12/2022
Introduction
Tropaeolum majus L. (Figure 1) is popularly known as garden nasturtium, nasturtium, Indian cress, or monks cress and belongs to the Tropaeolaceae family. It is a native plant of the Andes, mainly from Bolivia and Colombia where it grows wild, however, it was brought to Europe from Peru in the 16th century and is successfully cultivated as an annual decorative plant (1,2). It is currently found in all of Europe, and in some regions of Africa, Asia and Oceania (3).

This species is used in folk medicine to treat several diseases. Its leaves are used to treat asthma, urinary tract infections, cardiovascular disorders, and constipation (4). Pre-clinical studies have shown antihypertensive and diuretic effects (5,6) and in vitro assays using culture cells revealed anti-adipogenic effects of T. majus extracts (7). In addition, several toxicologic studies (chronic and subchronic toxicity, reproductive toxicity, and genotoxicity) have been conducted and demonstrated that T. majus infusions and aqueous extracts are safe (8,9). However, the use of high doses of hydroethanolic extracts should not be recommended to men of reproductive age and to pregnant women because high doses of these extracts (> 300 mg/kg) have interfered with reproductive function and gestation of animals (4,8-10). Pre-treatment with T. majus methanolic alcohol extract provides protection against diethyl maleate-induced blood and liver toxicity in rats, with results confirmed by histological examinations (11). In recent years, T. majus flower has been widely used in culinary as unconventional food plant (UFP) to decorate plates, especially salads, and is characterized by a spicy flavour and as an important source of carotenoids (antheraxanthin, zeaxanthin, lutein, β-cryptoxanthin, zeinoxanthin, α-carotene, β-carotene, violaxanthin) and phenolic compounds (quercetin, myricetin, kaempferol, pelargonidin, delphinidin, cyanidin, derivatives of hydroxycinnamic acid) (12-16).
Terms such as "functional foods" or "nutraceuticals" are widely used in the marketplace. These foods are regulated by the Food and Drug Administration (FDA) under the authority of the Federal Food, Drug and Cosmetic Act, although they are not specifically defined by law. Thus, functional foods are not officially recognised as a regulatory category by the FDA in the United States and the same is true in Europe, where Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods applies. "General function" claims under Article 13.1 of the EC Nutrition and Health Claims Regulation refer to the role of a nutrient or substance in growth, development and bodily functions; psychological and behavioural functions; slimming and weight control, satiety or reduction of available energy from the diet. T. majus may be a candidate for a general function claim if a cause-effect relationship is demonstrated for a particular physiologic effect.
Considering its spontaneous worldwide use as an unconventional food plant based on a perceived presumption of safety by the consumer and supported by published information, an exploratory investigation was carried out to evaluate, for the first time, the acceptance of T. majus flowers in a diet by adding it in different plates, as well as their effects on corporal composition, biochemical and haematological parameters in healthy volunteers.
Materials and methods
Participants
The study was made with the voluntary participation of six healthy individuals, both sexes (one man and five women), ages between 21 and 71 years old (mean 52.50 ± 17.28 years old) recruited randomly from a convenience population asked to participate in sensorial tests by the researcher team. The acceptance prior to the study and adherence during the study of T. majus flowers in the diet were assessed through questionnaires in which the volunteers described their opinions on the visual and taste aspects of the prepared meals. The following recipes were included in the acceptance study before the test: soup, meat pie, roast, bread, cod, rice, juice, sweet pie, and gelatin. The parameters questioned were: i) Have you ever heard of unconventional food plants (UFPs)? ii) Do you usually consume (UFPs)? iii) Have you ever tasted this plant? After tasting, how do you describe it? iv) You can use the suggested terms to define the meals: Appearance: pleasant/unpleasant/other; Color; Odor: intense/mild/other; Flavor: bitter/sweet/spicy/other; Texture: velvety/grainy/liquid/pasty/other. All procedures observed the principles of good clinical practices from the Helsinki Declaration and respective amendments (17). The volunteers were included in the study after informed written consent. The inclusion criteria were the acceptance of nasturtium taste as pleasant and willingness to include it in their daily diet for one month. The following exclusion criteria were considered: (i) any possibility of pregnancy, (ii) men of reproductive age, (iii) any pre-existing disease. Additionally, the participants filled out an Informed Consent Form to indicated if they smoked and if they took any medications, and, if so, which ones.
Procedures
Firstly, the volunteers selected in accordance with the criteria described above were given detailed information about the objectives, methods, and their role in the project. After signing informed written consent, data were collected for all participants by applying a semiquantitative food frequency questionnaire (FFQ), already validated for a Portuguese population (18) defining a mean reference portion consumed over one year for all food groups. The questionnaire used was composed of a list of the nutrients belonging to the seven conventional food table/wheel groups (19) and one closed section with five categories of the frequencies of consumption of twelve high-rated items (dairy products, fatty-, lean- and cod- fish, white and red meat, olive oil, whole meal bread and cereals, eggs, sweets, vegetables and legumes, and fruits). Thereafter, the volunteers received the recipes for the preparation of the meals containing T. majus flowers. The ingestion was divided into three meals per day (breakfast, lunch and dinner) such that 20 g of flowers were ingested daily for 30 days. The portion of 20 g per day divided into the main meals was based on the recommendation for intake of salad leaves regularly used by the population (for example, lettuce, rocket), which is a minimum of 12-15 leaves per day (20).
Meals were diversified, for example, salads, soups, cakes, and sandwiches, among other foods. At two time points, one at time zero (before starting the consumption of T. majus) and the second at the end of the study, the body composition of all volunteers were evaluated by dual-energy x-ray absorptiometry (DXA Lunar Prodigy Advance - GE Healthcare, Chicago, Illinois, USA). The parameters measured were Body Fat Percentage (BFP), Visceral Adipose Tissue (VAT), and Subcutaneous Adipose Tissue (TAS). Heart rate and arterial pressure were also measured, and blood and urine samples were collected for clinical analysis (hematological and biochemical) in the Laboratory LEB (Lisbon, Portugal).
During the study, participants were asked for each meal: i) Did you like/dislike the addition of the T. majus flowers? Justify. ii) Do you think it could become a regular option in your diet or not? Justify. In addition, the volunteers were contacted daily by the researchers to report whether they had eaten the entire daily portion or if there were leftovers to quantify (in scoops), and to register their opinion about the meals, as well as to report any unwanted/unexpected effects.
Plant Material
The plant material was collected on 12-July, 2021, at Latitude 38.955834, Longitude - 8.994359, Dr Luis César Pereira Urban Park, Time: 14.40 h. The manual harvest was conducted by one technician and the sample was identified and deposited by the Curator of Vascular Plants, Herbário LISU - Jardim Botânico/Herbarium LISU - Botanical Garden, Museu Nacional de História Natural e da Ciência, Lisboa, Portugal (Voucher number: LISU270425). The material was provided fresh to the volunteers, separated in portions of 20 g (daily portion) and kept at -20 oC until consumption.
Statistics
Data are reported as mean ± standard error of the mean (SEM) were compared by T-test using GraphPadPrism 5® software (GraphPad software, San Diego, CA, USA). A p value <0.05 was considered significant in all experiments.
Results and Discussion
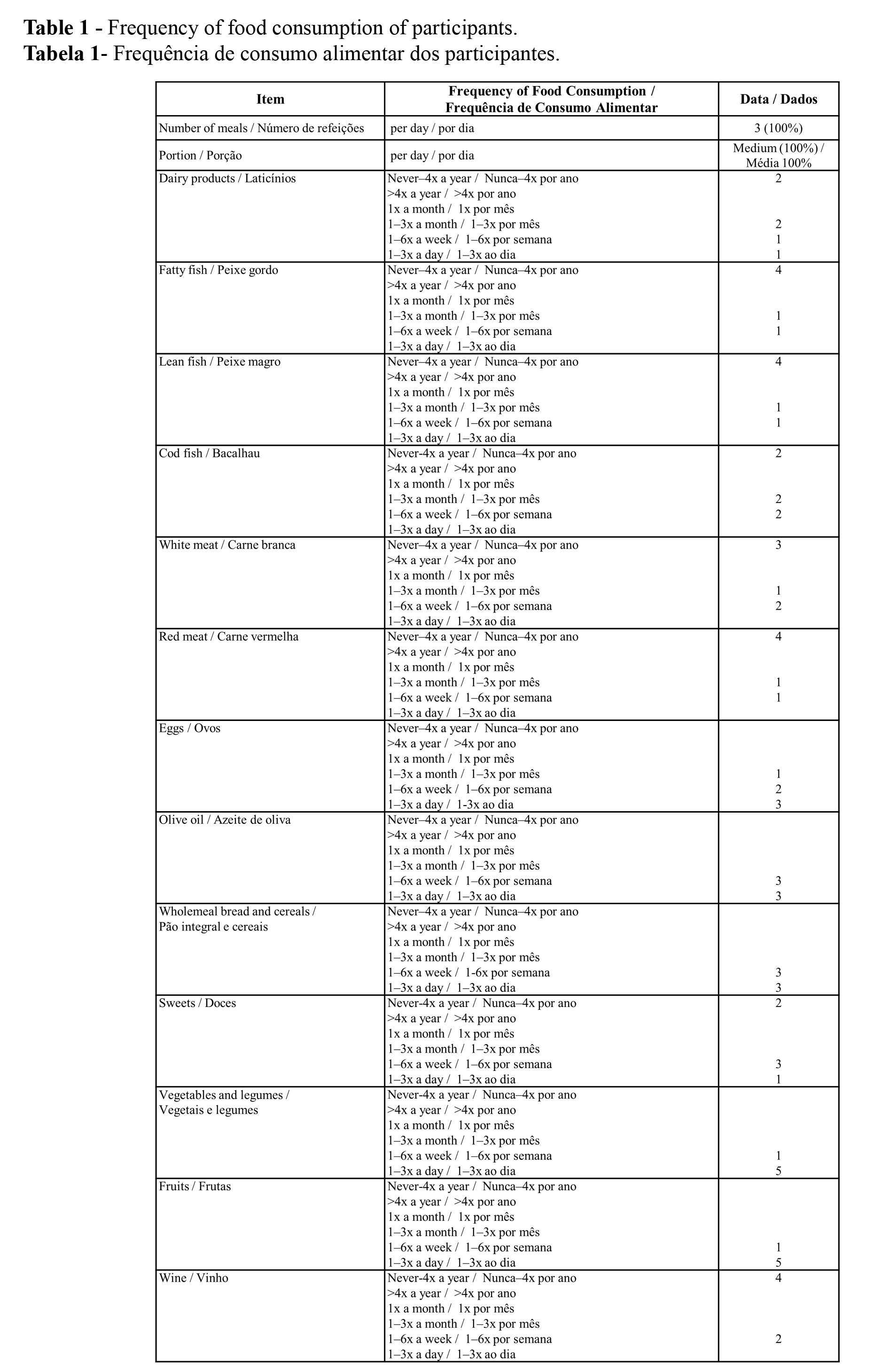
A healthy diet intended to control the risk of obesity was identified for the study participants based on their dietary habits, characterized by abundant and variable consumption of plant foods, high consumption of cereals, olive oil as the main fat, low intake of red meat, and null to moderate consumption of wine (Table 1). It is well reported that the high consumption of red meat, saturated fatty acids, and cholesterol may be associated with increased risk of diabetes and mortality due to cardiovascular diseases (CVDs) (21-23). Moreover, Alzheimer’s disease protection has been associated with a higher intake of vegetables, fruit, whole grains, fish, and legumes and with a lower intake of high-fat dairy, processed meat, and sweets (24). Epidemiological studies suggest a role of fruits and vegetables in protection against disease risks and aging (25), and for this reason the WHO considers that these should be the main foods to be ingested.
The analysis of parameters related to the body composition revealed that there was no observed effect on Body Fat Percentage (BFP), Visceral Adipose Tissue (VAT), and Subcutaneous Adipose Tissue (TAS) after T. majus ingestion (Figure 2).
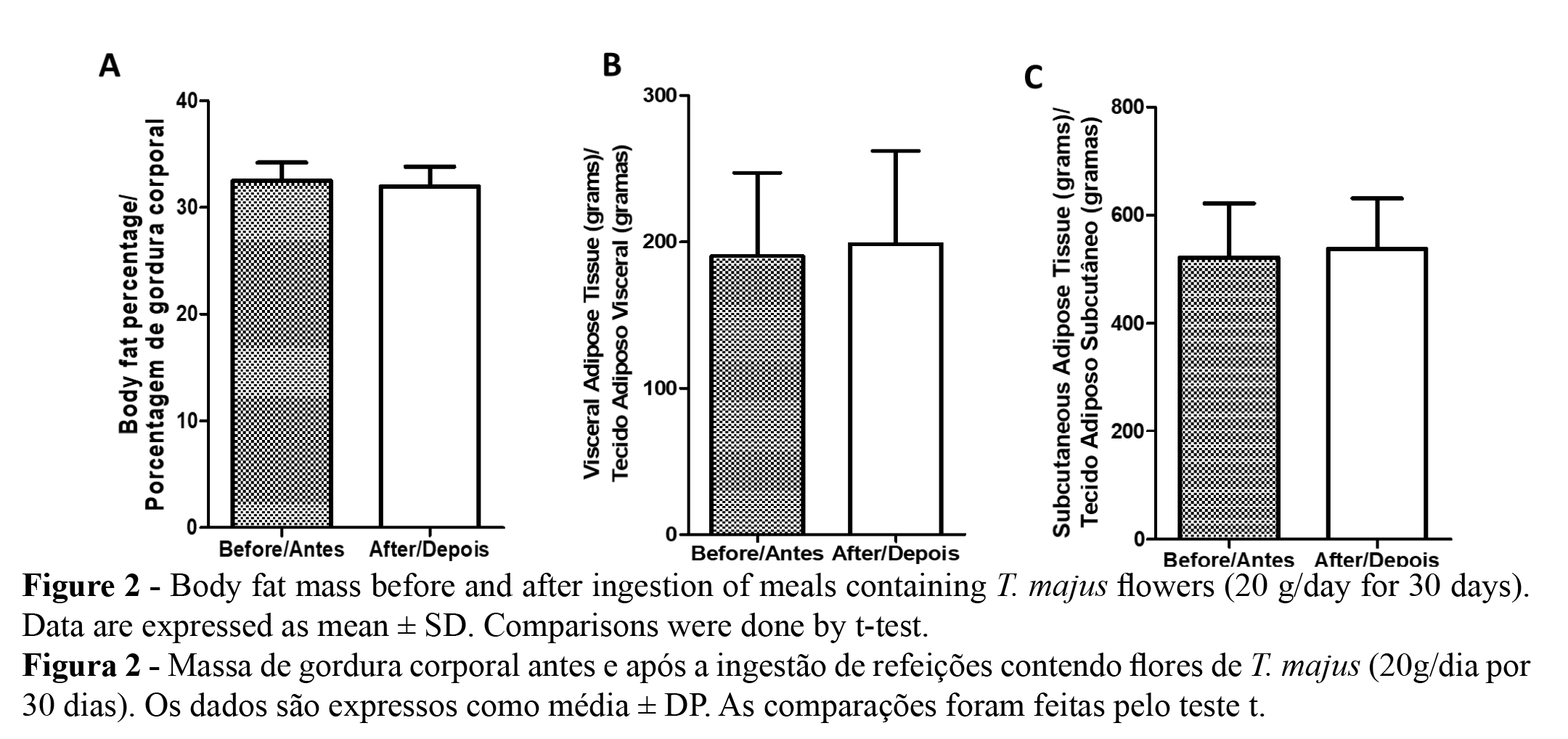
Kim et al. (7) demonstrated in cell culture (3T3-L1 adipocytes) that ethanolic extract of T. majus decreases lipid accumulation and inhibits the expression of peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding proteins (CEBPs) and sterol regulatory element-binding transcription factor 1 (SREBF1), which are transcription factors involved in the regulation of the adipogenesis pathway in 3T3-L1 adipocytes. In the present study, neither an effect on lipid accumulation nor a significant reduction in the rate of blood triglyceride content resulting from intake of 20 g daily of T. majus flowers was observed. The results are likely not relatable and concern very different study models and experimental conditions but raise interest in future studies with an intake of T. majus by participants with a higher percentage of fat mass over a longer period of time.
All participants reported that meals prepared using the daily portion of 20 g of T. majus flowers had a pleasant taste and no participant left the study. Mlcek et al., (27) also reported good acceptance of T. majus in a study that involved sensory evaluation of several species of edible flowers. In addition, during daily contact with the researchers responsible by study, no volunteer reported any changes in the number of bowel movements and stool consistency, urinary volume, or any other discomfort or unexpected change/symptom during the 30 days period of T. majus flowers ingestion and within two weeks after study completion. Likewise, no alterations were observed in rate heart and arterial pressure.
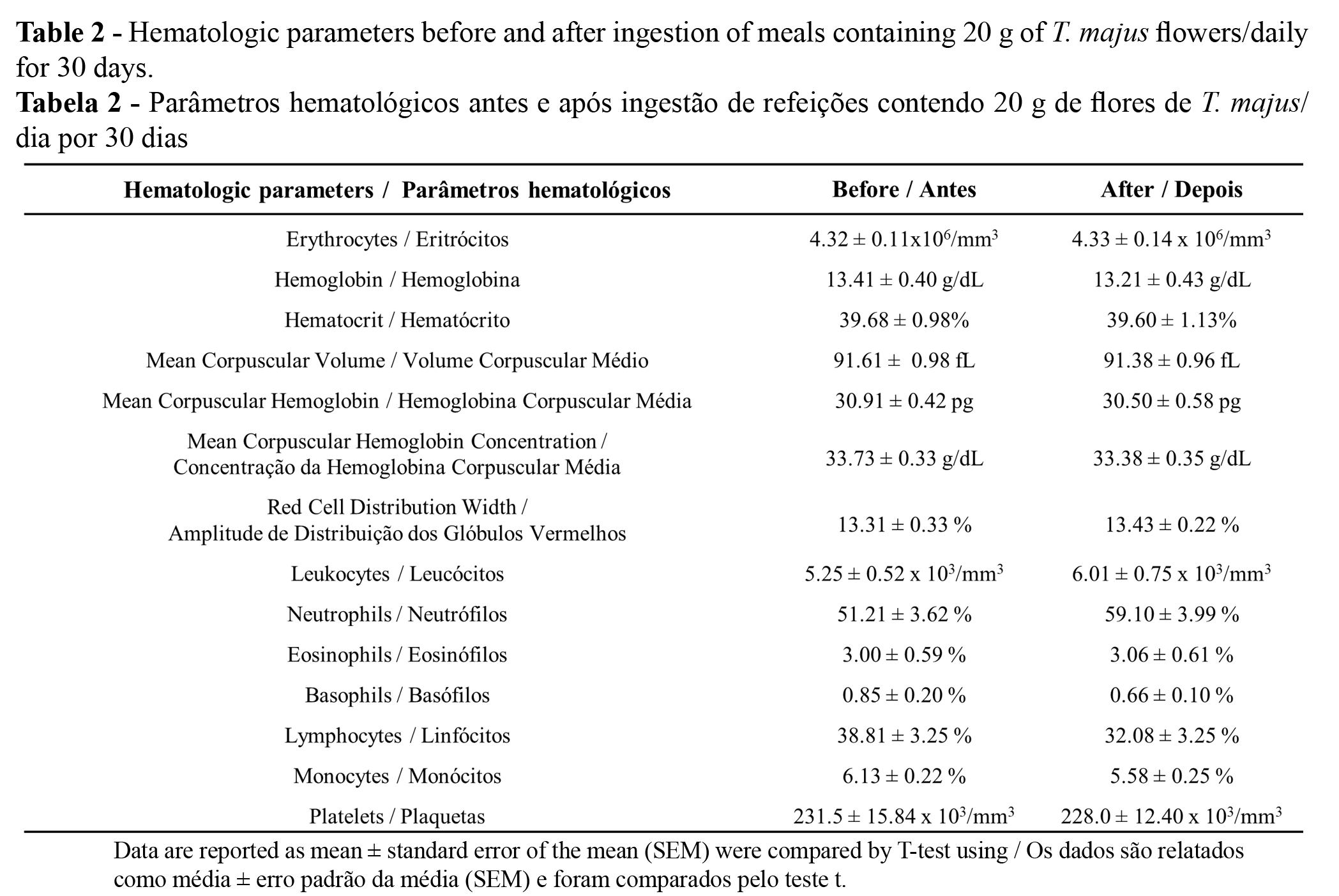
No changes were found when the hematological parameters were analyzed (Table 2), nor within the evaluated serum biochemical parameters used to assess liver, kidney, pancreas and metabolic functions (Table 3). The absence of toxicity of T. majus was formerly demonstrated by Araújo et al. (8), in a preclinical study using hydroethanolic extract by 90-day in rodents and lagomorphs. No changes in renal parameters, such as serum urea and serum creatinine, were also reported by Gasparoto Junior et al. (4), using ethanolic extract of T. majus and one of its main constituents, isoquercetin. Only for the biomarker C-Reactive Protein (CRP) a significant decrease was observed after adding T. majus flowers to the diet (Table 3) from 1.18 ± 0.16 mg/dL to 0.73 ± 0.04 mg/dL although in the range of healthy levels. It is well known that CRP is an acute-phase protein that has been associated with response to injury and systemic inflammation (28). Nowadays, epidemiological studies have shown a consistent association between cardiovascular disease risk and CRP concentrations. Elevated levels of CRP are related to inflammatory processes which are related to vascular cell activation, thrombosis, and accumulation of lipids and atherosclerotic process (29). T. majus contains a variety of bioactive compounds which are also known for their anti-inflammatory and antioxidant properties, including ascorbic acid (Vitamin C), flavonoids, carotenoids, and other polyphenolics (30). Furthermore, aqueous extracts of T. majus significantly suppressed the production of inflammatory mediators such as TNF-alpha, Prostaglandin E2 (PGE2), and leukotriene LTB4 in human blood cells (31). Another group of interesting substances from T. majus are the thermostable glucosinolates (32,33), such as glucotropaeolin, which is metabolized into benzyl isothiocyanate and has significant antitumor activity (34, 35). Thus, monitoring CRP before and after ingestion of T. majus for a longer time period and within a more representative group of human volunteers may contribute to a better understanding of the reason and significance of the decrease observed in our study.
Only about 103 plant species are responsible for 90% of the world's food supply despite estimates showing that there are about 27,000 plant food species (36). Hence, there are many species with alimentary potential which are neglected. Many of these species were used in the past, but their use has been abandoned with the industrialization and urbanization processes. In the last years, several of these species, including T. majus, have been highlighted as nutritious UFPs with high impact in Latin- and Ibero-American countries, namely Brazil and Portugal (12). Thus, more studies on its acceptance, safety, and nutritional value are important to understand any benefit/risk relationship for its use in the human diet.
The European Green Deal has the "Farm to Fork" strategy as a central focus to make food systems fair, sustainable, and healthy. The role of UFPs in this path is indisputable, but increasing the adoption of healthy and sustainable diets does not mean adhering to all the available UFPs offered without any criteria (37). Hence, the need to anticipate emerging risks for edible flower consumption has been brought to the EFSA´s (European Food Safety Authority) agenda. The guarantee of clear information is a consumer demand which shall be satisfied, a purpose that should unite universities/researchers and authorities, applying the Qualified Presumption of Safety (QPS) approach for the safety assessment of botanicals and botanical preparations published by EFSA (38). As an example of UFPs approach, we recall that EFSA was asked very recently by the European Commission whether there are duly reasoned safety objections to the placing on the market dried flowers of Clitoria ternatea L. as a traditional food (TF), known as butterfly pea, from a third country within the European Union (39). EFSA noted the in vitro haemolytic and cytotoxic effects reported for some cyclotides (circular proteins) as well as data indicating possible effects on the immune system, and uterus (although these cyclotides responsible for these effects have not been detected in Clitoria ternatea). Given the potential exposure to cyclotides resulting from the use of C. ternatea for the preparation of herbal infusions and the unknown toxicological profile of the cyclotides present in these edible flowers, the EFSA considered that could have some risk to human health. Thus, EFSA raised safety objections to the placing on the EU market of the dried flowers of Clitoria ternatea.
Considering the UFP T. majus, the a priori guidance of the EFSA for the safety assessment of plants and botanical preparations intended to be used as ingredients in food supplements applies (40), which provides that plants or botanical preparations for which an adequate body of knowledge exists can benefit from a 'presumption of safety (QPS)' without the need for further testing. In this situation, the QPS decision for T. majus can be based on available data on the history of safe use at traditional exposure levels, i.e., data regarding the safety of aqueous extracts of T. majus and consumption of the whole flower, with no reports of adverse effects (41).
In the particular case of T. majus flowers, there is evidence for the acceptable safety of its use as a wholly natural ingredient or dietary supplement in daily meals, well supported by studies of aqueous extracts (typical culinary medium) in animals and here in humans for the first time. However, since there are warnings in the literature about potential additional concerns related to the reproductive effects of ethanol extracts, it is advisable that further research should be carried out in search of a substance or group of substances for which a value should be defined as a limit related to observable health effects. Taking into account that the existing quality and toxicity data are insufficient to derive such a value, and T. majus concentrates obtained in alcohol seem unsuitable for unrestricted food use, the safety assessment of T. majus should be further developed for its various extracts.
Finally, it is important to recognize that, as this is a pilot exploratory work, there are some limitations in this study: 1) the limited number of participants (six) whose recruitment has been harmed by a study in pandemic phase; 2) the heterogeneous distribution of sex and age of the participants; 3) the lack of a control group and 4) lack of estimation of energy and nutrient intake, although the frequency of consumption and food portion size were already evaluated.
Conclusion
Results obtained indicate that T. majus flowers are well accepted when added to different meals and corroborate the information in the literature about its use being safe for humans. This is the first exploratory study referring to the safety of consumption of T. majus flowers in healthy humans, alerting to the importance of designing additional, more complete studies to confirm the promising results in a larger number of volunteers.
Authors' Contributions Statement
SFA, MCC, MGLS, and RGT undertook the experimental procedures. SFA, RGT, APS, LMR, and MCC executed and discussed the statistical analysis and wrote and corrected the manuscript in its final version.
Acknowledgements
This research is funded by Fundação para a Ciência e a Tecnologia (FCT) through grant UIDB/04567/2020 to CBIOS. Sérgio Faloni de Andrade is funded by Foundation for Science and Technology (FCT) - Scientific Employment Stimulus contract with the reference number CEEC/CBIOS/PMHD/2018.
Conflict of Interests
Editors involved in this manuscripts’ authorship had no participation in the review or decision process. All authors have stated that there are no financial and/or personal relationships that could represent a potential conflict of interest.
References
- Jakubczyk, K., Janda, K., Watychowicz, K., Łukasiak, J., & Wolska, J. (2018). Garden nasturtium (Tropaeolum majusL.) - a source of mineral elements and bioactive compounds. Rocz Panstw Zakl Hig, 69(2), 119-126.
- Tropaeolum majus: info from PIER (PIER species info). (2021). Retrieved 7 December 2021, from http://www.hear.org/pier/species/tropaeolum_majus.htm
- Tropaeolum majusL. (2021). Retrieved 7 December 2021, from https://www.gbif.org/species/2889934
- Gomes, C., Lourenço, E., Liuti, É., Duque, A., Nihi, F., & Lourenço, A. et al.(2012). Evaluation of subchronic toxicity of the hydroethanolic extract of Tropaeolum majusin Wistar rats. Journal Of Ethnopharmacology, 142(2), 481-487. doi: https://10.1016/j.jep.2012.05.023
- Gasparotto Junior, A., Gasparotto, F., Lourenço, E., Crestani, S., Stefanello, M., & Salvador, M. et al. (2011). Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majusL.: Evidence for the inhibition of angiotensin converting enzyme. Journal of Ethnopharmacology, 134(2), 363-372. doi: https://10.1016/j.jep.2010.12.026
- Barboza, L., Prando, T., Dalsenter, P., Gasparotto, F., Gasparotto, F., & Jacomassi, E. et al. (2014). Prolonged Diuretic Activity and Calcium-Sparing Effect of Tropaeolum majus: Evidence in the Prevention of Osteoporosis. Evidence-Based Complementary And Alternative Medicine, 2014, 1-6. doi: https://10.1155/2014/958291
- Kim, G., Kim, J., Kim, G., & Choi, S. (2017). Anti-adipogenic effects of Tropaeolum majus(nasturtium) ethanol extract on 3T3-L1 cells. Food & Nutrition Research, 61(1), 1339555. doi: https://10.1080/16546628.2017.1339555
- Araújo, V., Andreotti, C., Reis, M., de Lima, D., Pauli, K., & Nunes, B. et al. (2018). 90-Day Oral Toxicity Assessment of Tropaeolum majusL. in Rodents and Lagomorphs.Journal Of Medicinal Food, 21(8), 823-831. doi: https://10.1089/jmf.2017.0128
- Traesel, G., Machado, C., Tirloni, C., Menetrier, J., dos Reis Lívero, F., & Lourenço, E. et al.(2017). Safety Assessment and Botanical Standardization of an Edible Species from South America. Journal of Medicinal Food, 20(5), 519-525. doi: https://10.1089/jmf.2016.0143
- Khorsandi, L., & Oroojan, A. (2018). Toxic effect of Tropaeolum majusL. leaves on spermatogenesis in mice. JBRA Assisted Reproduction. doi: https://10.5935/1518-0557.20180035
- Koriem, K., Arbid, M., & El-Gendy, N. (2010). The protective role of Tropaeolum majuson blood and liver toxicity induced by diethyl maleate in rats. Toxicology Mechanisms And Methods, 20(9), 579-586. doi: https://10.3109/15376516.2010.518171
- Mazon, S., Menin, D., Cella, B., Lise, C., Vargas, T., & Daltoé, M. (2020). Exploring consumers’ knowledge and perceptions of unconventional food plants: case study of addition of Pereskia aculeataMiller to ice cream. Food Science And Technology, 40(1), 215-221. doi: https://10.1590/fst.39218
- Garzón, G., & Wrolstad, R. (2009). Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chemistry, 114(1), 44-49. doi: https://10.1016/j.foodchem.2008.09.013
- Mlcek, J., & Rop, O. (2011). Fresh edible flowers of ornamental plants – A new source of nutraceutical foods. Trends In Food Science & Technology, 22(10), 561-569. doi: https://10.1016/j.tifs.2011.04.006
- Navarro-González, I., González-Barrio, R., García-Valverde, V., Bautista-Ortín, A., & Periago, M. (2014).Nutritional Composition and Antioxidant Capacity in Edible Flowers: Characterisation of Phenolic Compounds by HPLC-DAD-ESI/MSn. International Journal Of Molecular Sciences, 16(1), 805-822. doi: https://10.3390/ijms16010805
- Niizu, P., & Rodriguez-Amaya, D. (2006). Flowers and Leaves of Tropaeolum majusL. as Rich Sources of Lutein. Journal Of Food Science, 70(9), S605-S609. doi: https://10.1111/j.1365-2621.2005.tb08336.x
- World Medical Association Declaration of Helsinki.(2013). JAMA, 310(20), 2191.
- Lopes, C., Aro, A., Azevedo, A., Ramos, E., & Barros, H. (2007).Intake and Adipose Tissue Composition of Fatty Acids and Risk of Myocardial Infarction in a Male Portuguese Community Sample. Journal of the American Dietetic Association, 107(2), 276–286. https://doi.org/10.1016/j.jada.2006.11.008
- Roda dos Alimentos, PNPAS. (2022). Retrieved 2 January 2022, from ttps://alimentacaosaudavel.dgs.pt/roda-dos-alimentos/
- Silva, C.L. (2011). Repositório Institucional da UnB: Página inicial. https://repositorio.unb.br/bitstream/10482/9899/1/2011_ClislianLuziaSilva.pdf
- Giacosa, A., Barale, R., Bavaresco, L., Gatenby, P., Gerbi, V., & Janssens, J. et al.(2013). Cancer prevention in Europe. European Journal Of Cancer Prevention, 22(1), 90-95. doi: 10.1097/cej.0b013e328354d2d7
- Da Silva, A., Valente, A., Chaves, C., Matos, A., Gil, A., & Santos, A. et al.(2018). Characterization of Portuguese Centenarian Eating Habits, Nutritional Biomarkers, and Cardiovascular Risk: A Case Control Study.Oxidative Medicine And Cellular Longevity, 2018, 1-10. doi: 10.1155/2018/5296168
- Pereira da Silva, A., Costa, M., Aguiar, L., Matos, A., Gil, Â., & Gorjão-Clara, J. et al.(2020). Impact on Longevity of Genetic Cardiovascular Risk and Lifestyle including Red Meat Consumption. Oxidative Medicine And Cellular Longevity, 2020, 1-14. doi: 10.1155/2020/130541324.
- Mosconi, L., Murray, J., Davies, M., Williams, S., Pirraglia, E., & Spector, N. et al. (2014). Nutrient intake and brain biomarkers of Alzheimer's disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study.BMJ Open, 4(6), e004850-e004850. doi: 10.1136/bmjopen-2014-004850
- Virmani, A., Pinto, L., Binienda, Z., & Ali, S. (2013).Food, Nutrigenomics, and Neurodegeneration—Neuroprotection by What You Eat!. Molecular Neurobiology, 48(2), 353-362. doi: 10.1007/s12035-013-8498-3
- WHO, “Diet, nutrition and the prevention of chronic diseases,” World Health Organ Technical Report Series, vol. 916, pp. 1–149, 2003
- Mlcek, J., Plaskova, A., Jurikova, T., Sochor, J., Baron, M., & Ercisli, S. (2021).Chemical, Nutritional and Sensory Characteristics of Six Ornamental Edible Flowers Species. Foods, 10(9), 2053. https://doi.org/10.3390/foods10092053
- Yao, Z., Zhang, Y., & Wu, H. (2019). Regulation of C-reactive protein conformation in inflammation. Inflammation Research, 68(10), 815-823. doi: 10.1007/s00011-019-01269-1
- Avan, A., Tavakoly Sany, S., Ghayour‐Mobarhan, M., Rahimi, H., Tajfard, M., & Ferns, G. (2018). Serum C‐reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. Journal Of Cellular Physiology, 233(11), 8508-8525. doi: 10.1002/jcp.26791
30 Dujmović, M., Radman, S., Opačić, N., Fabek Uher, S., Mikuličin, V., Voća, S., & Šic Žlabur, J. (2022). Edible Flower Species as a Promising Source of Specialized Metabolites. Plants, 11(19), 2529. https://doi.org/10.3390/plants1119252
- Tran, H., Márton, M., Herz, C., Maul, R., Baldermann, S., Schreiner, M., & Lamy, E. (2016). Nasturtium (Indian cress, Tropaeolum majus nanum) dually blocks the COX and LOX pathway in primary human immune cells. Phytomedicine, 23(6), 611-620. doi: 10.1016/j.phymed.2016.02.025
- Breme K, Fernandez X, Meierhenrich UJ, Brevard H, Joulain D. Identification of new, odor-active thiocarbamates in cress extracts and structure-activity studies on synthesized homologues. J Agric Food Chem. 2007 Mar 7;55(5):1932-8. doi: 10.1021/jf062856e. Epub 2007 Feb 2. PMID: 17269787
- Barba, F. J., Nikmaram, N., Roohinejad, S., Khelfa, A., Zhu, Z., & Koubaa, M. (2016). Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Frontiers in Nutrition, 3. https://doi:10.3389/fnut.2016.00024
- Soundararajan P, Kim JS. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules. 2018; 23(11):2983. https://doi.org/10.3390/molecules23112983
- Platz, S., Kühn, C., Schiess, S., Schreiner, M., Kemper, M., Pivovarova, O., Pfeiffer, A. F. H., & Rohn, S. (2015). Bioavailability and metabolism of benzyl glucosinolate in humans consuming Indian cress (Tropaeolum majusL.). Molecular Nutrition & Food Research, 60(3), 652–660. https://doi.org/10.1002/mnfr.201500633
- Leal, M., Alves, R., & Hanazaki, N. (2018). Knowledge, use, and disuse of unconventional food plants. Journal Of Ethnobiology And Ethnomedicine, 14(1). doi: 10.1186/s13002-018-0209-8
- The European Green Deal: opportunities to anticipate and address emerging risks. (2022). Retrieved 2 January 2022, from https://summitdialogues.org/dialogue/18790
- EFSA (European Food Safety Authority), (2014). Scientific Opinion on a Qualified Presumption of Safety (QPS) approach for the safety assessment of botanicals and botanical preparations. EFSA Journal, 12(3). doi: https://10.2903/j.efsa.2014.3593
- EFSA (European Food Safety Authority), (2022). Notification of dried flowers of Clitoria ternateaL. as a traditional food from a third country pursuant to Article 14 of Regulation (EU) 2015/2283. EFSA supporting publication 2022:EN-7084. 17pp. doi:10.2903/sp.efsa.2022.EN-7084 ISSN: 2397-8325
- EFSA (European Food Safety Authority), (2009). Guidance on Safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA Journal, 7(9). doi: https://10.2903/j.efsa.2009.1249
- EFSA Scientific Committee, More, S.J., Bampidis, V., Benford, D., Bragard, C., Halldorsson, T.I., Hernandez-Jerez, A.F., Hougaard, B.S., Koutsoumanis, K.P, Machera, K., Naegeli, H., Nielsen, S.S., Schlatter, J.R., Schrenk, D., Silano, V., Turck, D., Younes, M.., Gundert-Remy, U., Kass, G.E.N., Kleiner, J., Rossi, A.M., Serafimova, R., Reilly, L. and Wallace, H.M. (2019). Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA Journal;17(6):5708, 17 pp. https://doi.org/10.2903/j.efsa.2019.5708
